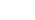Cell line development (CLD) is a critical yet time-consuming step in biomanufacturing. It typically requires substantial resources to expand and screen hundreds or even thousands of single-cell clones. After transfection, cell pools must fully recover before growth, productivity, and product quality are characterized to identify the optimal pool for single-cell cloning (SCC). In biomedical research—including drug development, cancer studies, and gene function analysis—cell line screening plays a vital role. Stable cell lines are widely used in the production of antibodies and recombinant protein drugs, gene and cell therapy development, disease modeling, gene function research, and drug target discovery.
I. Traditional Cell Line Construction Workflow
-
Cell Selection & Preparation
-
Cell Line Selection: Choose appropriate cell types (e.g., HEK293, HeLa, CHO) or primary cells based on the research goals, ensuring reliable sourcing and clear lineage.
-
Culture Condition Optimization: Maintain cells in optimal conditions (medium, serum, temperature, CO₂) with strict standardization.
-
Cell Authentication: Use STR (short tandem repeat) analysis to confirm identity and eliminate cross-contamination.
-
-
Gene Editing Tool Design
-
Editing Tools: Common options include CRISPR/Cas9, TALEN, or ZFN; CRISPR/Cas9 is the most widely used due to its efficiency and simplicity.
-
sgRNA Design: Use online tools (e.g., CRISPR Design, Benchling) to design specific sgRNAs and minimize off-target effects.
-
Donor Template: For knock-in applications, design a homology-directed repair template with 500–1000 bp homology arms.
-
-
Transfection & Editing
-
Transfection Methods: Choose based on cell type—lipofection, electroporation, or viral vectors (e.g., lentivirus, adenovirus for difficult-to-transfect cells).
-
Efficiency Validation: 48–72 hours post-transfection, extract genomic DNA and assess editing using T7E1 assay, sequencing, or Surveyor assay.
-
-
Screening & Cloning
-
Selection Markers: Use antibiotics (e.g., puromycin, G418) or fluorescent proteins (e.g., GFP, RFP) to enrich edited cells.
-
Single-Cell Cloning: Isolate clones by limiting dilution or FACS; limiting dilution requires seeding 0.5–1 cell/well and culturing for 2–3 weeks.
-
Clone Expansion: Expand promising clones step by step.
-
-
Gene Editing Validation
-
Genomic Level: Confirm knock-in/knock-out by PCR and sequencing.
-
Transcription Level: Use qPCR to assess mRNA expression.
-
Protein Level: Use Western blot or immunofluorescence to detect target protein.
-
-
Functional Validation
-
Phenotype Analysis: Design functional assays based on goals (e.g., proliferation, apoptosis, migration).
-
Stability Test: Pass cells for at least 10 generations to confirm genetic stability.
-
-
Cryopreservation
-
Cryoprotectant: Use freezing medium containing 10% DMSO and 90% FBS.
-
Freezing Protocol: Freeze at 1×10⁶ cells/mL with a -1°C/min cooling rate to -80°C, then transfer to liquid nitrogen.
-
Recovery Test: Confirm viability and target gene expression upon thawing.
-
-
Data Recording & Standardization
-
Documentation: Log all procedures and parameters (e.g., passage number, transfection conditions).
-
SOPs: Develop standard operating procedures for repeatable results.
-
Traditional Cell Line Development Process
II. Limitations of Traditional Cell Line Development
-
Time & Cost Intensive
The process is complex, lengthy, and relies on costly reagents and equipment. -
Random Integration
Foreign gene insertion is rare and random, with variable expression due to chromosomal context—necessitating extensive screening for stable expression. -
Low Success Rate
Minor errors in protocol can cause failure, leading to high overall costs and low efficiency.
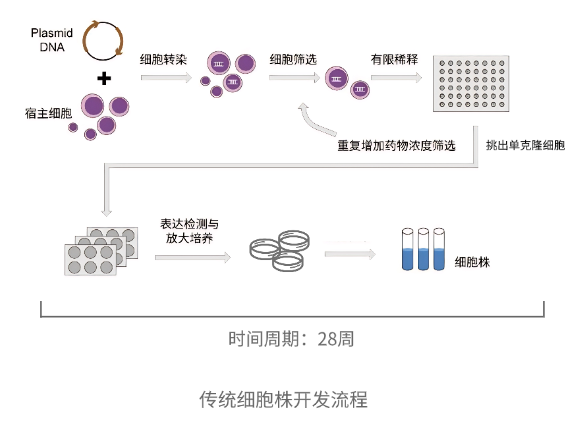
Challenges such as time consumption, randomness, and low success rates continue to hinder researchers. As biotech tools advance, many automated solutions are emerging. The OptoBot®1000 Single-Cell Optoelectronic Microfluidic System by Optoseeker is specifically designed to overcome these bottlenecks.
Comparison Between Traditional Cell Line Development Process and the 0ptoBot®1000 Single-Cell Photoelectric Microfluidic System Workflow
III. Advantages of OptoBot®1000
-
High-Efficiency Single-Cell Cloning
OptoBot®1000 utilizes microfluidic chip technology to isolate single cells within a sealed environment. It enables pre-screening of clones based on growth and productivity before amplification. Compared to traditional limiting dilution or FACS, it significantly improves cloning efficiency. -
Accelerated Timelines
With precise single-cell manipulation and an integrated CLD workflow, high-yielding clones can be identified within just five days. -
Resource Saving
By screening cells for growth and secretion before expansion, the system reduces the number of clones that need to be cultured, saving time and consumables. -
Higher Success Rate
The precise isolation and screening process significantly reduces experimental failure and improves clone success rates. -
Automation & Standardization
OptoBot®1000 offers fully automated workflows and standardized protocols, ensuring reproducibility and consistency across experiments.
The OptoBot®1000 minimizes resource consumption in clone screening and expansion, enabling the selection of high-performance clones even under low cell viability conditions. It supports downstream applications in drug discovery and antibody development. With its efficiency, stability, and traceability, OptoBot®1000 helps researchers save significant time and labor while ensuring data reliability—empowering next-generation cell line development.