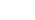In medical diagnostics and disease management, rapid and accurate protein biomarker detection is critical. Conventional methods like ELISA offer accuracy but suffer from lengthy procedures (2-3 hours), operational complexity, and high costs. Professor Zhang Shuailong and Fu Rongxin’s team at Beijing Institute of Technology recently unveiled a breakthrough in Nano Letters: an electroosmotic-driven digital optofluidics (e-DOF) platform. This innovation quantifies protein-binding nanoscale deformations via hyperspectral interferometry, slashing detection time to 15 minutes with 0.21 nM sensitivity (50× improvement over ELISA) and 100% clinical accuracy.
OptoSeeker’s proprietary digital microfluidics system provided technical support and key components, enabling this leap in efficiency and sensitivity.

I. Protein Detection: Challenges & Opportunities
Protein biomarkers (e.g., IgM for acute infections, IgG for immune history) are vital for early diagnosis and treatment monitoring. Current methods face limitations:
-
Lateral Flow Assays (LFA): Low-cost but qualitative/semi-quantitative with limited sensitivity.
-
ELISA: Gold standard yet slow (2-3 hrs), operator-dependent, and sample-intensive (100s of μL).
-
Advanced techniques (LSPR/SERS): Sensitivity gains offset by expensive substrates and complex workflows.
II. Core Innovation: e-DOF Technology
1. Label-Free Optical Detection
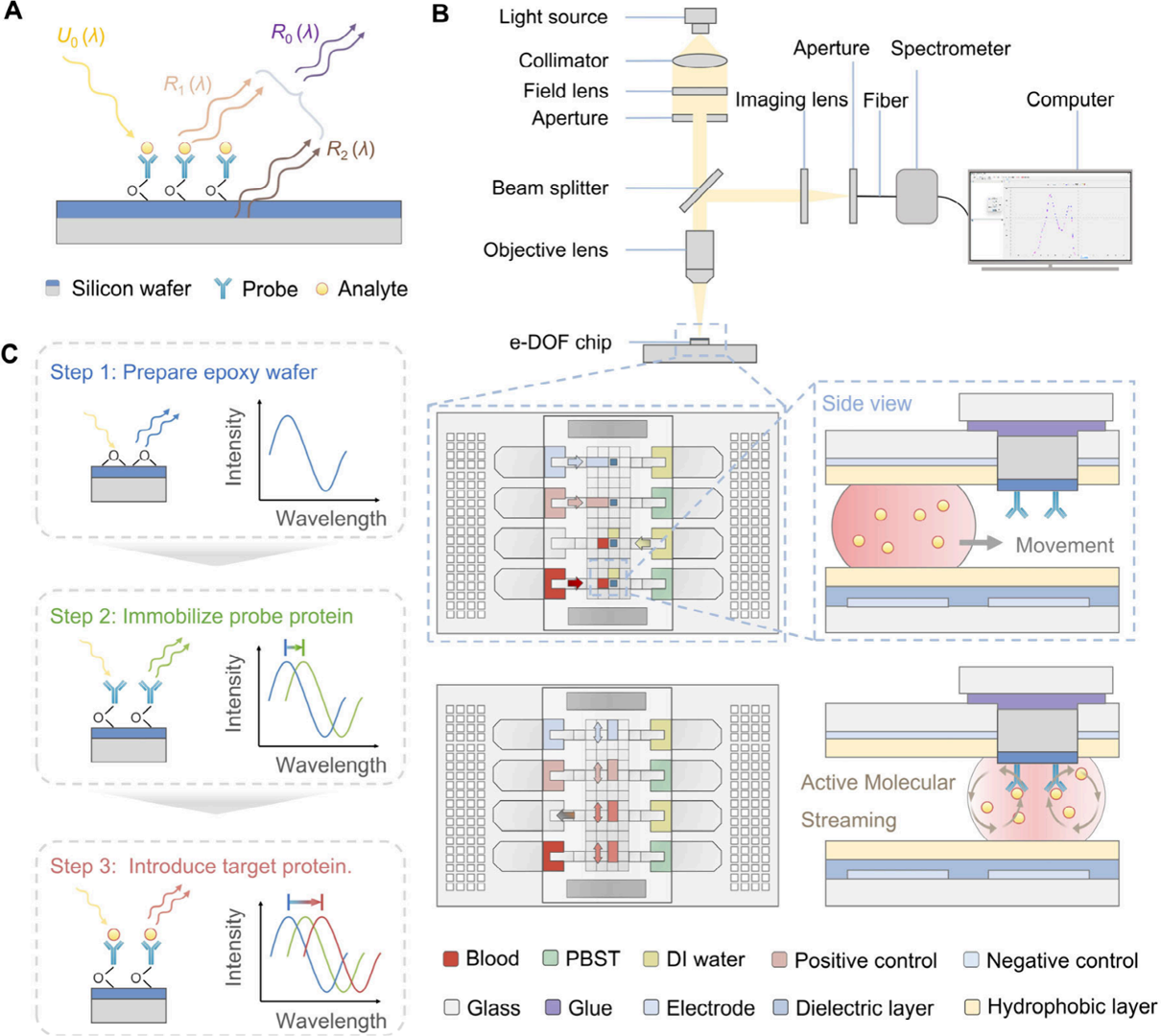 Figure 1: Schematic diagram of system architecture and detection principle
Figure 1: Schematic diagram of system architecture and detection principle
A self-interfering photonic chip (Fig 1A) measures nanoscale protein-binding deformations via phase shifts in reflected interference spectra. Fast Fourier Transform (FFT) analysis eliminates enzymatic labeling and secondary antibodies (Fig 1C).
2. Electroosmotic Molecular Cycling
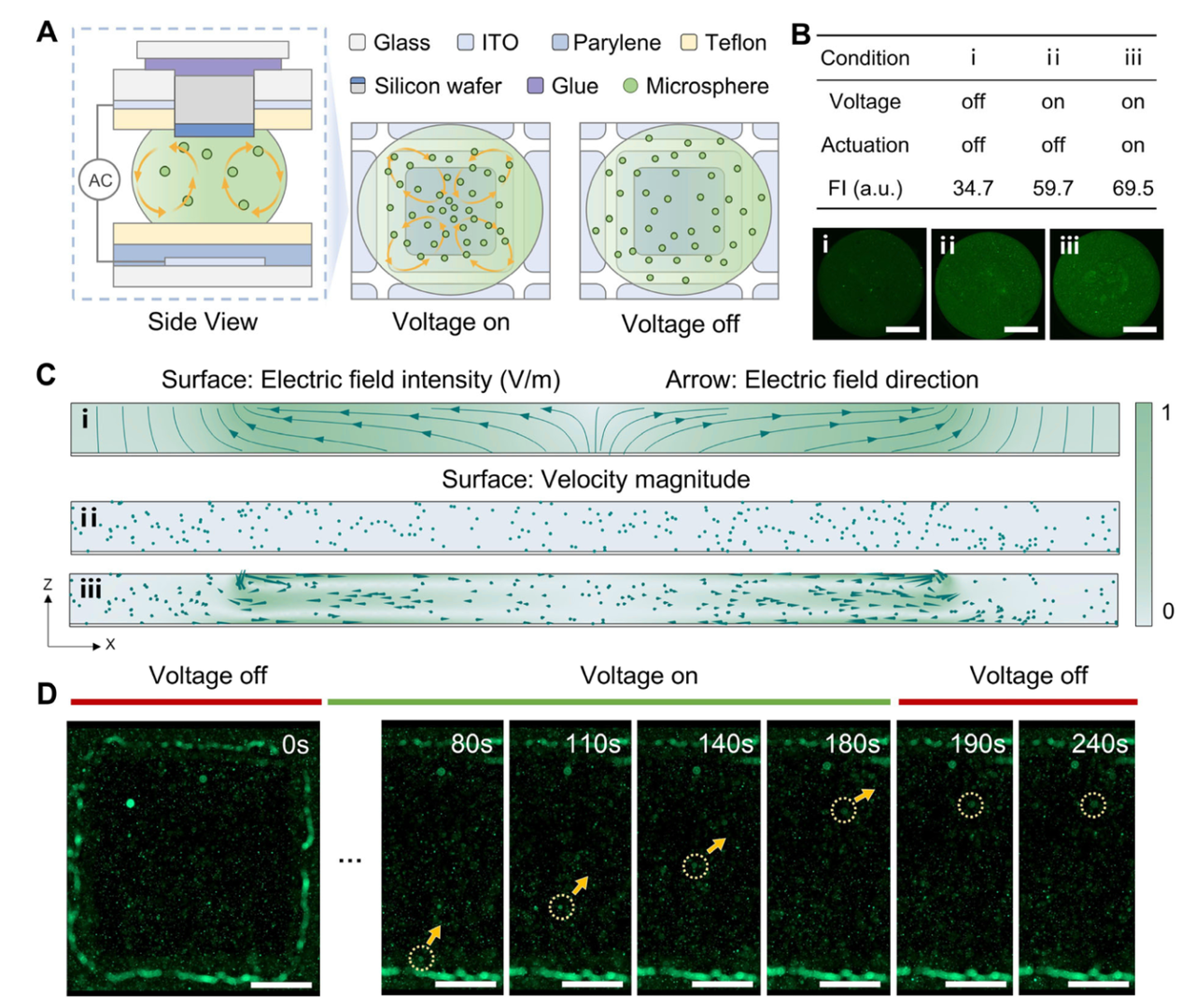 Figure 3: Electroosmotic flow-induced circulation within microfluidic droplets
Figure 3: Electroosmotic flow-induced circulation within microfluidic droplets
Electric fields generate directional molecular flow within droplets (Fig 3A/C), doubling reaction efficiency (fluorescence intensity: 34.7 → 69.5, Fig 3B). Target-probe binding completes in 15 minutes vs. conventional hours.
III. Performance Validation
1.Sensitivity
 Figure 2: Characterization, condition optimization and quantitative performance via hyperspectral interferometry
Figure 2: Characterization, condition optimization and quantitative performance via hyperspectral interferometry
LOD = 0.21 nM (R²=0.94, Fig 2E), outperforming fluorescence at low concentrations (Fig 2C).
2.Clinical Accuracy
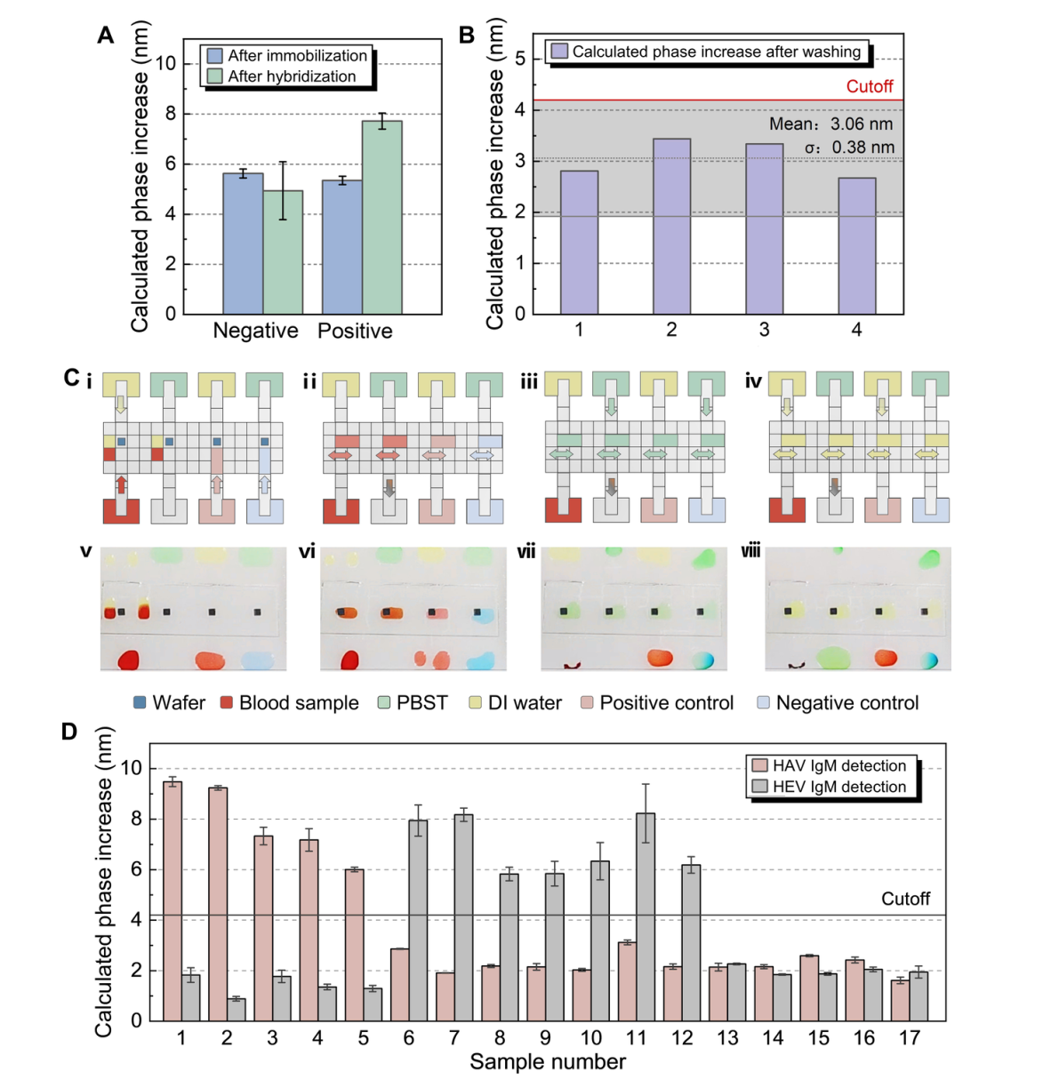
Figure 4: Chip detection performance and qualitative detection of clinical samples
100% concordance with ELISA for HAV/HEV IgM in 17 samples (Fig 4D), using 4 μL volume.
3.Whole-Blood Compatibility
Reduced nonspecific adsorption enables future POCT applications (Fig 4B).
IV. Competitive Advantages
| Parameter | e-DOF Platform | ELISA |
|---|---|---|
| Time | 15 min | 2-3 hrs |
| Sample Volume | 4 μL | 200-500 μL |
| Sensitivity | 0.21 nM | 10.5 nM |
| Automation | Full | Manual steps |
V. Future Applications
-
Multiplexed Detection: Integrated probes for simultaneous biomarker screening.
-
Precision Medicine: Real-time therapeutic monitoring.
-
Global Health: Infectious disease screening in resource-limited settings.
This Nano Letters study pioneers the fusion of microfluidics and optical sensing. e-DOF enables rapid, automated, low-cost protein detection, shifting diagnostics from central labs to point-of-care.
Contact for purchasing or trial opportunities.